TEXAS LEADS THE NATION: WARNING LABELS ON ADDITIVES
On June 22, 2025, Governor Greg Abbott signed Senate Bill 25 (SB25), known as the Make Texas Healthy Again Act. Beginning January 1, 2027, Texas will require prominent on-pack warning labels whenever food sold in the state contains any of 44 specific additives—including synthetic colorants like Red 40, Yellow 5, Blue 1, titanium dioxide, bleached flour, and partially hydrogenated oils. The mandated label must declare the following:
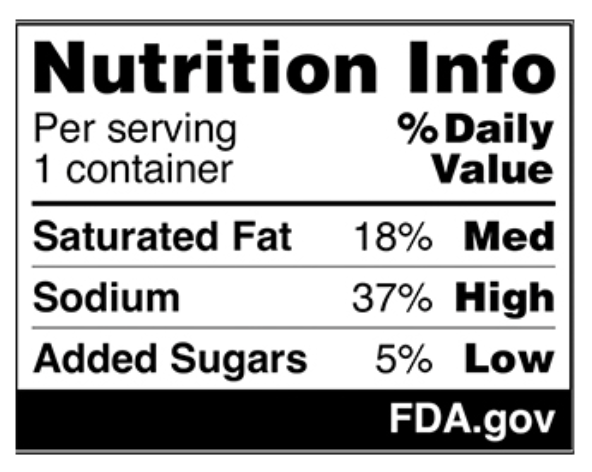
"WARNING: This product contains an ingredient that is not recommended for human consumption by the appropriate authority in Australia, Canada, the European Union, or the United Kingdom."
These warnings must appear in font no smaller than the existing ingredient list, in a visible, high-contrast placement, and also be included on product webpages when sold online. The list of additives spans a broad range of chemicals used in ultra-processed foods, from food dyes to emulsifiers, preservatives, and bleached and bromated flours. While many are legal in the U.S., advocates cite regulatory caution in other nations and studies linking ingredients like titanium dioxide to health concerns. SB25 marks the first U.S.state to mandate additive-specific warning labels, shifting from nutrition-focused warnings to ingredient-level transparency. The law ties into a broader movement spearheaded by U,S, Health and Human Services Secretary, Robert F. Kennedy Jr., who’s backing state-level action through his “Make America Healthy Again” campaign.

New Texas law demands warning labels on additives like Red 40 and Yellow 5, found in popular cereals like this.
SB25 calls for action in four key domains:
- Labeling Additives — Enforce warning labels on any food product with listed ingredients by 2027.
- Education — Introduce nutrition education in all grade schools (K–8), including daily physical activity mandates. Also include nutrition electives in high school and add nutrition coursework for universities to retain state funding.
- Medical Training — Require continuing nutrition and metabolic-health training for licensed professionals (doctors, nurses, PA’s) to maintain licensure.
- Advisory Committee — Create a seven-member Texas Nutrition Advisory Committee to study connections between additives, ultra-processed foods, and chronic disease; they’ll also guide curriculum development.
Consumer advocacy groups like Consumer Reports and the Environmental Working Group view this as a major win for transparency and public health. They argue that Texas is stepping in where the federal government lagged.
Critics, including industry groups like the Consumer Brands Association, label the law “misleading” and legally questionable. They contend the foreign recommendations are often recommendations rather than bans, and that the law could drive unnecessary costs, legal action, and consumer confusion. The Texas Attorney General may seek injunctions or impose daily fines up to $50,000 per non‑compliant product.
Federal Preemption & Company Strategies
SB 25 includes a federal preemption clause: if the FDA or USDA declares a listed ingredient safe, prohibits it, or mandates similar warning labels, Texas’s requirement on that ingredient would be invalidated. This ensures companies won't face conflicting obligations, but also opens interpretative challenges.
Manufacturers face a choice: label, reformulate, or litigate. Many may reformulate products to remove additives to avoid the label. Others might issue Texas‑specific packaging or challenge the law in court before enforcement begins. Given Texas's national influence, some companies may extend changes nationwide to avoid logistical complexity.
What starts in Texas doesn’t stay in Texas. New labeling laws could ripple nationwide as companies react to SB 25.
What’s Next—and What to Watch
- Rulemaking Phase: Texas regulations detailing enforcement are to be finalized by December 31, 2025, in advance of the January 1, 2027 effective date.
- Legal Pressure: Industry groups may file lawsuits, citing First Amendment and federal preemption concerns.
- Federal Response: If the FDA acts—e.g., banning titanium dioxide or creating front-of-pack labels—federal rules could override Texas’s law.
- Consumer Impact: Parents, schools, and health professionals should begin awareness preparation. Early label updates may appear before 2027, and companies might release natural‑color versions of iconic products.
In Summary
Texas’s SB 25 is a game‑changer: the first U.S. state to mandate bold warning labels for specific food additives tied to foreign caution. Extending far beyond packaging, it rethinks school PE requirements, nutrition education, and health‑care training. Backed by a federal health initiative, this law may pressure both industry and regulators toward broader transparency, public‑health focus, and ingredient refinement—whether in Texas or across the nation.
As the Warnings Doctor, I fully support the Texas law and hope the Federal Government, through the FDA, comes through with sensible national guidelines and even requirements. Putting politics aside, the health of our nation should be a top priority of our government.